ADSORPSI ION SIANIDA DALAM LARUTAN MENGGUNAKAN ADSORBEN HIBRIDA AMINOPROPIL SILIKA GEL DARI SEKAM PADI TERIMPREGNASI ALUMINIUM (Adsorption of Cyanide Ions in Solution Using a Hybrid Adsorbent Aminopropyl Silica Gel from Rice Husks of Impregnated With)
Amaria Amaria(1*)
(1) Jurusan Kimia FMIPA Universitas Negeri Surabaya Kampus Unesa Ketintang Surabaya Gedung C3 Lt 1, Surabaya
(*) Corresponding Author
Abstract
ABSTRAK
Telah dibuat dua macam adsorben hibrida aminopropil silika gel yang terimpregnasi aluminium (APSG-Al) dan silika gel terimpregnasi aluminium (SG-Al) dari silika gel sekam padi sebagai bahan untuk adsorpsi ion sianida dalam larutan. Interaksi antara adsorben dengan ion sianida dalam larutan dilakukan dalam sistem batch. Parameter-parameter yang dikaji dalam penelitian ini adalah pengaruh pH medium, pengaruh waktu interaksi dan pengaruh konsentrasi awal ion sianida terhadap kemampuan adsorpsi adsorben hibrida amino silika gel terimpregnasi aluminium. Analisis kuantitatif ion-ion sianida yang tersisa di dalam filtrat diuji dengan alat elektroda selektif ion. Data hasil pengaruh waktu interaksi dianalisis dengan model kinetika adsorpsi, data hasil pengaruh konsentrasi ion sianida dianalisis dengan model isoterm adsorpsi Langmuir dan Freundlich. Di samping itu gugus fungsional yang diperkirakan terlibat dalam adsorpsi diidentifikasi dengan spektrofotometer infra merah dan kristalinitas adsorben diuji dengan defraksi sinar X.
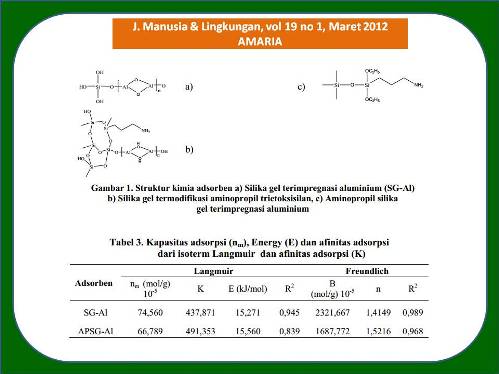
Hasil penelitian menunjukkan bahwa hasil identifikasi spektroskopi infra merah menunjukkan adsorben APSG-Al memiliki gugus silanol (Si-OH), siloksil (Si-O-Si), gugus amina primer, NH2. Hasil analisis XRD nilai 2θ pada 65,51 menunjukkan bahwa aluminium yang terimpregnasi pada silika berbentuk alumina Al2¬O3. Hasil adsorpsi ion sianida oleh hibrida aminopropil silika gel terimpregnasi aluminium (APSG-Al) menunjukkan adsorpsi sianida terjadi maksimum pada pH 5 sebesar 67,62 %, sedangkan SG-Al mengadsorpsi sianida secara maksimum pada pH 8 sebesar 51,11%. Kajian kinetika dari pengaruh waktu interaksi menunjukkan bahwa adsorben APSG-Al maupun SG-Al memiliki konstanta laju adsorpsi k1 masing-masing adalah 2,7. 10-3 dan 1,9.10-3 min-1. Data kapasitas adsorpsi menunjukkan bahwa adsorben APSG-Al dan SG-Al cenderung mengikuti model isoterm adsorpsi Freundlich.
ABSTRACT
This research has made two kinds of adsorbents, namely hybrid aminopropil silica gel from rice husk that has been impregnated with aluminum (APSG-Al) and silica gel impregnated with aluminum (SG-Al) of rice husk silica gel as the material for the adsorption of cyanide ions in solution. The interaction between the adsorbent with cyanide ions in solution performed in a batch system. The parameters examined in this study were the influence of medium pH, the effect of interaction time and the effect of initial concentration of cyanide ion adsorption ability of adsorbent hybrid amino silica gel impregnated with aluminum. Quantitative Analysis of cyanide ions left in the filtrate was tested by means of ion selective electrode. The effect of interaction time data were analyzed with kinetic model, the data of the influence of cyanide ion concentration was analyzed by Langmuir adsorption isotherm model and Freundlich. The results showed that the infrared spectroscopic identification results show APSG-Al adsorbent has silanol groups (Si-OH), siloxil (Si-O-Si), primary amine group, NH2. The result of XRD analysis of the price of 2θ at 65.51 indicates that the aluminum impregnated with the silica in the form of alumina Al2O3. The result of adsorption of cyanide by the hybrid silica gel impregnated with aluminum aminopropil (APSG-Al) showed maximum adsorption occurred at pH 5 was 67.62%, silica gel impregnated with aluminum was 51,11%. Study the kinetics of the effect of interaction time showed that the adsorbent APSG-Al and SG-Al-Al has the adsorption rate constant k1 is 2.7, 10-3and 1.9,10-3 min-1, respectively. Adsorption equilibrium data showed that the adsorbent APSG-Al and SG-Al tend to follow the adsorption isotherm model Freundlich.
Keywords
Full Text:
Artikel lengkap (PDF) (Bahasa Indonesia)Article Metrics
Refbacks
- There are currently no refbacks.
Copyright (c) 2017 Jurnal Manusia dan Lingkungan







