Rutaceae: conservation at Eka Karya Bali Botanic Garden and its in vitro antifungal activity screening
I Putu Agus Hendra Wibawa(1), Arrohmatus Syafaqoh Li'aini(2*), Putri Sri Andila(3), Frelyta Ainuz Zahro'(4)
(1) Eka Karya Bali Botanic Garden, Research Center for Plant Conservation and Botanic Gardens, Indonesian Institute of Sciences Candikuning, Baturiti, Tabanan, Bali 82191
(2) Eka Karya Bali Botanic Garden, Research Center for Plant Conservation and Botanic Gardens, Indonesian Institute of Sciences Candikuning, Baturiti, Tabanan, Bali 82191, Indonesia
(3) Eka Karya Bali Botanic Garden, Research Center for Plant Conservation and Botanic Gardens, Indonesian Institute of Sciences Candikuning, Baturiti, Tabanan, Bali 82191, Indonesia
(4) Faculty of Agriculture, Universitas Brawijaya, Jl. Veteran, Ketawanggede, Lowokwaru, Malang, East Java 65145, Indonesia
(*) Corresponding Author
Abstract
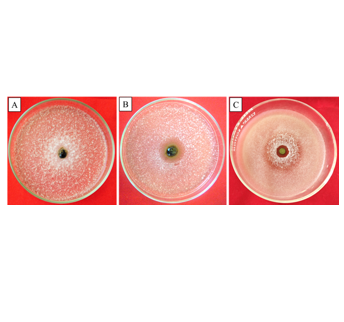
Several species of Rutaceae have been widely used and commercialized in all regions in Indonesia. Some species of Rutaceae are consumed as fresh fruit and traditional medicine for various kinds of diseases, as well as to add aroma to various Indonesian culinary. Since 1959, Eka Karya Bali Botanic Garden (Eka Karya BBG) has successfully collected dozens of Rutaceae species with unknown potential. In addition to reporting the conservation of Rutaceae in Eka Karya BBG, this study aimed to screen the antifungal activity of Rutaceae methanolic extract toward Aspergillus niger, Cladosporium sp., and Fusarium solani. Leaves of 13 species of Rutaceae (Boenninghausenia sp., Citrus aurantifolia, C. maxima, C. medica, Clausena sp., Melicope sp., Micromelum sp., Murraya paniculata, Toddalia sp., Zanthoxylum sp., Z. alatum, Z. limonella, and Z. ovalifolium) were collected, cleaned, air-dried, soaked in methanol for three days, then evaporated using a rotary evaporator to obtain the plant crude extract. The in vitro inhibitory assay was conducted by the diffusion method. As a result, only C. medica, Clausena sp., and Z. limonella exhibited antifungal activity against those tested fungi. Their antifungal activity increased on day 2 post-treatment but slowly decreased on day 3. Thus, the result of this experiment can be used as preliminary data to researchRutaceae plant extracts as an alternative method to control pathogenic fungi. However, further research is needed to maintain and increase its inhibitory effect.
Keywords
Full Text:
PDFReferences
Alwatban, M.A., Hadi, S., and Moslem, M.A. (2014). Mycotoxin production in Cladosporium species influenced by temperature regimes. J. Pure Appl. Microbiol., 8(6), pp. 4061-4069.
Adham, A.N. (2015). Phytochemical analysis and evaluation antibacterial activity of Citrus medica peel and juice growing in Kurdistan/Iraq. Journal of Applied Pharmaceutical Science, 5(10), pp. 136-141.
Andriastini, D.A., Ramona, Y., and Proborini, M.W. (2018). Hambatan in vitro cendawan antagonis pada Fusarium sp., penyebab penyakit pada tanaman buah naga (Hylocereus undatus (Haw.) Britton & Rose). Jurnal Metamorfosa, 5(2), pp. 224-233.
Apriliani, A., Sukarsa, and Hidayah, H.A. (2014). Kajian etnobotani tumbuhan sebagai bahan tambahan pangan secara tradisional oleh masyarakat di Kecamatan Pekuncen Kabupaten Banyumas. Scripta Biologica, 1(1), pp. 76–84.
Azwanida, N. (2015). A Review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants, 4(3), pp. 1-6.
Balakumar, S., Rajan, S., Thirunalasundari, T., and Jeeva, S. (2011). Antifungal activity of Aegle marmelos (L.) Correa (Rutaceae) leaf extract on dermatophytes. Asian Pasific Journal of Tropical Biomedicine, 1(4), pp. 309-312.
Cantín, C.M., Minas, I.S., Goulas, V., Jiménez, M., Manganaris, G.A., and Michailides, T.J. (2012). Sulfur dioxide fumigation alone or in combination with CO2-enriched atmosphere extends the market life of highbush blueberry fruit. Postharvest Biol. Technol., 67, pp. 84-91.
Chen, C., Chen, J., and Wan, C. (2020). Pinocembrin-7-Glucoside (P7G) reduced postharvest blue mold of navel orange by suppressing Penicillium italicum growth. Microorganisms, 8(4), pp. 1-17.
Chen, C.J., Li, Q.Q., Ma, Y.N., Wang, W., Cheng, Y.X., Xu, F.R., and Dong, X. (2019). Antifungal effect of essential oils from five kinds of Rutaceae plants - avoiding pesticide residue and resistance. Chemistry & Biodiversity, 16(4), pp. e1800688.
Costa, S.S., Arumugam, D., Gariepy, Y., Rocha, S.C.S., and Raghavan, V. (2013). Spilanthol extraction using microwave: calibration curve for gas chromatography. Chem. Eng. Trans., 32, pp. 1783-1788.
Crespo P., Gin´e-Bordonaba, J., Terry, L.A., and Carlen, C. (2010). Characterisation of major taste and health-related compounds of four strawberry genotypes grown at different Swiss production sites. Food Chem., 122(1), pp. 16-24.
Devi, A.D., Singh, T.C., Devi, O.I., Singh, S.S., Singh, A.R., and Singh, E.J. (2015). Phytochemical analysis of some traditional aromatic plant species of Thobal District, Manipur. Asian Journal of Pharmaceutical Science & Technology, 5(1), pp. 50-53.
Elumalai, K. and Kasinathan, I.D. (2016). Antioxidant activity and phytochemical screening of different solvent extracts Cluasena excavate Burm.f. (Rutaceae). MOJ Ecology & Environmental Science, 1(1), pp. 1-6.
Gopalasatheeskumar, K., Parthiban, S., Manimaran, T., and Boopathi, T. (2017). Phytochemical screening on various extracts (benzene, ethanolic, and aqueous) of stem parts of Zanthoxylum rhetsa (Roxb.) DC. International Journal of Universal Pharmacy and Bio Sciences, 6(2), pp. 79-91.
Hulyati, R., Syamsuardi, and Arbain, A. (2014). Studi etnobotani pada tradisi Balimau di Kota Pariaman, Sumatera Barat. Jurnal Biologi Universitas Andalas, 3(1), pp. 14-19.
Horváth, G., Bencsik, T., Ács, K., and Kocsis, B. (2016). Sensitivity of ESBL-producing gram-negative bacteria to essential oils, plant extracts, and their isolated compounds. In: K. Kon and M. Rai, eds., Antibiotic Resistance, 1st ed. Amsterdam: Academic Press, pp. 239-269
Howard, L, Clark, J., and Brownmiller, C. (2003). Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric., 83(12), pp. 1238-1247.
Jimenez-Garcia, S.N., Garcia-Mier, L., Feregrino-Perez, J., Garcia-Trejo, J.F., Ramirez-Gomez, X.S., Guevara-Gonzalez, R.G., and Feregrino-Perez, A.A. (2018). Fusarium mycotoxins and metabolites that modulate their production, In: Askun, T., ed., Fusarium: Plant Diseases, Pathogen Diversity, Genetic Diversity, Resistance and Molecular Markers, 1st ed. London, UK: IntechOpen. pp 23-40.
Jiménez-Reyes, M.F., Carrasco, H., Olea, A.F., and Silva-Moreno, E. (2019). Natural compounds: a sustainable alternative to the phytopathogens control. J. Chil. Chem. Soc., 64(2), pp. 4459-4465.
Kalariya, M.V., Prajapati, R.P., and Chavda, J.R. (2019). Pharmacognostic and phytochemical evaluation of Bijapur (Citrus medica Linn.) fruit. Journal of Pharmacognosy and Phytochemistry, 8(3), pp. 4159-4164.
Kosh-Komba, E., Toumnou, L.A., Zinga, I., Touckia, I., Lembo, P.U.N.Z.W., Mukeina, G., Semballa, S., Yongo, O.D., and Syssa-Magale, J.L. (2017). Phytochemical screening, antifungal and antibacterial effect of Zanthoxylum zanthoxyloides and Zanthoxylum macrophylum used in traditional medicine in Yamboro (Central African Republic). European Journal of Medicinal Plants, 19(3), pp. 1-11.
Kruger, E, Dietrich, H., Schopplein, E., Rasima, E., and Kurbel, P. (2011). Cultivar, storage conditions and ripening effects on physical and chemical qualities of red raspberry fruit. Postharvest Biol. Technol., 60(1), pp. 31-37.
Kumar, R., Saha, A., and Saha, D. (2012). A new antifungal coumarin from Clausena excavata. Fitoterapia, 83(1), pp. 230-233.
Lushchak, V.I., Matviishyn, T.M., Husak, V.V., Storey, J.M., and Storey, K.B. (2018). Pesticide toxicity: a mechanistic approach. EXCLI J., 17, pp. 1101-1136.
Maarisit, W. and Lawani, M. (2020). Chemical investigation and antimicrobial activity of medicinal plant Toddalia asiatica Lam. Indones. J. Chem., 20(5), pp. 1025-1031.
Meliki, R.L. and Lovadi, I. (2013). Etnobotani tumbuhan obat oleh Suku Dayak Iban Desa Tanjung Sari Kecamatan Ketungau Tengah Kabupaten Sintang. Protobiont, 2(3), pp. 129-135.
Mertha, I.G., Agil, A.I., Ilhamdi, M.L., and Zulkifli, L. (2018). Pelatihan teknik pembuatan herbarium kering dan identifikasi tumbuhan berbasis lingkungan sekolah di SMAN 4 Mataram. Jurnal Pendidikan dan Pengabdian Masyarakat, 1(1), pp. 82–87.
Miller, A.B., Cates, R.G., Lawrence, M., Soria, J.A.F., Espinoza, L.V., Martinez, J.V., and Arbizu, D.A. (2014). The antibacterial and antifungal activity of essential oils extracted from Guatemalan medicinal plants. Pharm. Biol., 53(4), pp. 548-554.
Nanasombat, S. and Wimuttigosol, P. (2011). Antimicrobial and antioxidant activity of spice essential oils. Food Sci. Biotechnol., 20, pp. 45-53.
Nandan, M.P. and Vangalapati, M. (2015). Phytochemical analysis and effect of various parameters on the extraction yield of flavonoids from the Citrus medica L. peel extract. International Advanced Research Journal in Science, Engineering and Technology, 2(8), pp. 5-9.
Negi, J.S., Bisht, V.K., Bhandari, A.K., Singh, P., and Sundriyal, R.C. (2011). Chemical constituents and biological activities of the genus Zanthoxylum: a review. African Journal of Pure and Applied Chemistry, 5(12), pp. 412-416.
Njeru, S.N., Obonyo, M.A., Nyambati, S.O., and Ngari, S.M. (2015). Antimicrobial and cytotoxicity properties of the crude extracts and fractions of Premna resinosa (Hochst.) Schauer (Compositae): Kenyan traditional medicinal plant. BMC Complement. Altern. Med., 15, pp. 1-9.
Novitasari, Jayuska, A., and Wibowo, M.A. (2014). Bioaktivitas anti rayap minyak atsiri dari daun jeruk sambal (Citrus microcarpa Bunge) terhadap rayap tanah Macrotermes sp. JKK, 3(4), pp. 57-62.
Osman, K., Evangelopoulos, D., Basavannacharya, C., Gupta, A., McHugh, T.D., Bhakta, S., and Gibbons, S. (2012). An antibacterial from Hypericum acmosepalum inhibits ATP-dependent MurE ligase from Mycobacterium tuberculosis. Int. J. Antimicrob. Agents, 39(2), pp. 124-129.
Pagare, S., Bhatia, M., Tripathi, N., Pagare, S., and Bansal, Y.K. (2015). Secondary metabolites of plants and their role: overview. Current Trends in Biotechnology and Pharmacy, 9(3), pp. 293-304.
Panara, K., Joshi, K., and Nishteswar, K. (2012). A review on phytochemical and pharmacological properties of Citrus medica Linn. International Journal of Pharmaceutical & Biological Archives, 3(6), pp. 1292-1297.
Pavela, R. (2014). Limitation of plant biopesticides, In: Singh, D. (ed.). Advances in Plant Biopesticides. New Delhi: Springer, pp. 347-359.
Pinaria, A.G. and Assa, B.H. (2017). Jamur patogen tanaman terbawa tanah. 1st ed. Malang: Media Nusa Creative.
Restuccia, C., Conti, G.O., Zuccarello, P., Parafati, L., Cristaldi, A., and Ferrante, M. (2019). Efficacy of different citrus essential oils to inhibit the growth and B1 aflatoxin biosynthesis of Aspergillus flavus. Environmental Science and Pollution Research, 26(30), pp. 31263-31272.
Sah, A.N., Juyal, V., and Melkani, A.B. (2011). Antimicrobial activity of six different parts of the plant Citrus medica Linn. Pharmacogn. J., 3(21), pp. 80-83.
Slamet, A. and Andarias, S.H. (2018). Studi etnobotani dan identifikasi tumbuhan berkhasiat obat masyarakat Sub Etnis Wolio Kota Baubau Sulawesi Tenggara. Proceeding Biology Education Conference, 15(1), pp. 721-732.
Soesanto, L., Mugiastuti, E., Ahmad, F., and Witjaksono. (2012). Diagnosis lima penyakit utama karena jamur pada 100 kultivar bibit pisang. J. HPT Tropika, 12(1), pp. 36-45.
Supabphol, R. and Tangjitjareonkun, J. (2014). Chemical constituents and biological activities of Zanthoxylum limonella (Rutaceae): a review. Tropical Journal of Pharmaceutical Research, 13(12), pp. 2119-2130.
Truong, D.H., Nguyen, D.H., Ta, N.T.A., Bui, A.V., Do, T.H., and Nguyen, H.C. (2019). Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. Journal of Food Quality, 2019, pp. 1-9.
Walker, G.M. and White, N.A. (2018). Introduction to fungal physiology. In: K. Kavanagh, ed., Fungi: Biology and Applications, 3rd ed. USA: John Wiley & Sons, pp. 1-34
Weiss, E.A. (1997). Essential oil crops. 1st ed. Victoria, Australia: CAB International, pp. 417-435
Wibawa, I.P.A.H., Saraswaty, V., Kuswantoro, F., Andila, P.S., Wardhani, P.K., Tirta, I.G., and Sujarwo, W. (2019). A study of essential oil from an invasive Piper aduncum L. Jurnal Biologi Udayana, 23(2), pp. 50-58.
Wibawa, I.P.A.H., Saraswaty, V., and Sujarwo, W. (2019a). Studi potensi minyak atsiri daun Boenninghausenia albiflora (Hook.) Rchb. ex Meisn. di Kebun Raya Eka Karya Bali. Buletin Kebun Raya, 22(2), pp. 73-82.
Wibawa, I.P.A.H and Lugrayasa, I.N. (2020). Studi potensi antioksidan dan antimikroba ekstrak buah lempeni (Ardisia elliptica Thunb.). Widya Biologi, 11(2), pp. 109-117.
Widodo and Wiyono, S. (2012). Penyakit keriting daun pepaya yang disebabkan oleh Cladosporium cladosporioides. Jurnal Fitopatologi Indonesia, 8(1), pp. 28-29.
Yassir, M. and Asnah. (2019). Pemanfaatan jenis tumbuhan obat tradisional di Desa Batu Hamparan Kabupaten Aceh Tenggara. BIOTIK: Jurnal Ilmiah Biologi Teknologi dan Kependidikan, 6(1), pp. 17-34.
You, Q., Wang, B., Chen, F., Huang, Z., Wang, X., and Luo, P.G. (2011). Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem., 125(1), pp. 201-208.
Article Metrics
Refbacks
- There are currently no refbacks.
Ilmu Pertanian (Agricultural Science) ISSN 0126-4214 (print), ISSN 2527-7162 (online) is published by Faculty of Agriculture Universitas Gadjah Mada collaboration with Perhimpunan Sarjana Pertanian Indonesia (PISPI) and licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.











_2025_-_kecil_.png)
