Seed germination and growth of Joseph’s coat (Amaranthus tricolor L.) following exposure with Naphthalene-1-Acetic Acid (NAA) and 2,4-Dichlorophenoxyacetic Acid (2,4-D)
Hadyan Pratama Lutfi Ilmam(1*), Kumala Dewi(2)
(1) Faculty of Biology, Universitas Gadjah Mada
(2) Faculty of Biology, Universitas Gadjah Mada
(*) Corresponding Author
Abstract
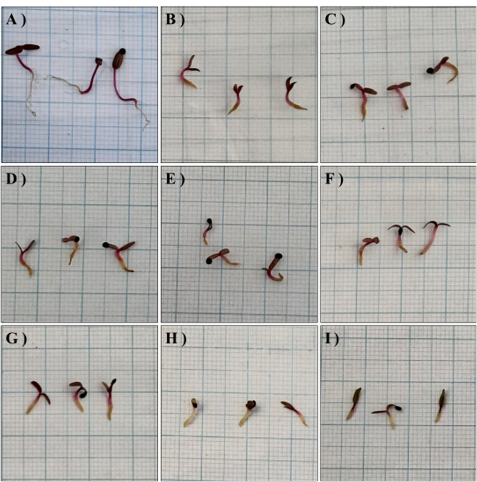
Amaranthaceae is a family of plants that can be used as vegetables and medicinal herbs. Amaranthus tricolor L. is commonly cultivated because it has fast growth rate and short life cycle that can be boosted by growth regulators such as auxins. A. tricolor L. is commonly cultivated because it has a fast growth rate and short life cycle. Growth regulators, such as auxins, can boost the growing process. This research aimed to study the effects of Naphthalene-1-Acetic Acid (NAA) and 2,4-Dichlorophenoxyacetic Acid (2,4-D) on the seed germination and growth of A. tricolor L. and to determine effective concentration of NAA or 2,4-D application to A. tricolor L. This research was arranged in a completely randomized design with exogenous hormones application as treatments. The treatments consisted of various concentrations of NAA and 2,4 D (0 ppm, 10 ppm, 20 ppm, 40 ppm, and 80 ppm) applied to A. tricolor L. plants every two weeks. Germination test of A. tricolor L. was carried out for 14 days, and the application of NAA and 2,4-D on A. tricolor L. plant was given for 56 days. Observations were made on the plant height, fresh and dry weight, stomatal density, and the content of chlorophyll and carotenoid. Data analysis was conducted using one-way analysis of variance and Duncan Multiple Range Test (DMRT) with significance level of 5%. NAA treatment delayed seed germination by one day compared to control, while 2,4-D treatment inhibited germination for several days with the higher concentration of 2,4-D applied, the greater inhibition of seed germination. NAA of 10 ppm increased plant height, fresh and dry weight, chlorophyll content, and leaf area of A. tricolor L. The application of NAA and 2,4-D reduced stomatal density and carotenoid content of A. tricolor L., with greater effects at higher concentrations of synthetic auxins. This research concluded that NAA or 2,4-D inhibited germination of A. tricolor L. seeds, NAA of 10 ppm effectively increased plant growth and chlorophyll content, but higher NAA concentrations inhibited growth. Application of 2,4-D with concentrations above 40 ppm could be lethal for A. tricolor L.
Keywords
Full Text:
PDFReferences
Agehara, S., Pride, L., Gallardo, M. and Hernandez-Monterroza, J. (2020). A Simple, Inexpensive, and Portable Image-Based Technique for Nondestructive Leaf Area Measurements. EDIS, 2020(6), pp. 1–6.
Ahammed, G. J. (2020). Role of ethylene crosstalk in seed germination and early seedling development A review. Plant Physiology and Biochemistry, 151, p. 8.
Arnon, D. I. (1949). Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24(1), pp.1–15.
Bala, V. C., Avid, M., Kumar, P. and Singh, S. (2019). A Review on Amaranthus Tricolor as a Traditional Medicinal Plant. World Journal of Pharmaceutical Research, 8 (11), p.13.
Balcerowicz, M. and Hoecker, U. (2014). Auxin – a novel regulator of stomata differentiation. Trends in Plant Science, 19(12), pp.747–749.
Butova, V. V., Bauer, T. V., Polyakov, V. A. and Minkina, T. M. (2023). Advances in nanoparticle and organic formulations for prolonged controlled release of auxins. Plant Physiology and Biochemistry, 201, 107808.
Campanoni, P. and Nick, P. (2005). Auxin-Dependent Cell Division and Cell Elongation. 1-Naphthaleneacetic Acid and 2,4-Dichlorophenoxyacetic Acid Activate Different Pathways. Plant Physiology, 137(3), pp.939–948.
Dayan, F. E. and Zaccaro, M. L. de M. (2012). Chlorophyll fluorescence as a marker for herbicide mechanisms of action. Pesticide Biochemistry and Physiology, 102(3), pp.189–197.
Dubois, M., Van den Broeck, L. and Inzé, D. (2018). The Pivotal Role of Ethylene in Plant Growth. Trends in Plant Science, 23(4), pp.311–323.
Flasiński, M. and Hąc-Wydro, K. (2014). Natural vs synthetic auxin: Studies on the interactions between plant hormones and biological membrane lipids. Environmental Research, 133, pp.123–134.
Gil, C. S., Jung, H. Y., Lee, C. and Eom, S. H. (2020). Blue light and NAA treatment significantly improve rooting on single leaf-bud cutting of Chrysanthemum via upregulated rooting-related genes. Scientia Horticulturae, 274, 109650.
Hendry, G. A. F. and Grime, J. P. (1993). Stress indicators: chlorophylls and carotenoids. In: Methods in Comparative Plant Ecology: A Laboratory Manual. 1st ed. Dordrecht : Springer. pp.48–152.
Hermanns, A. S., Zhou, X., Xu, Q., Tadmor, Y. and Li, L. (2020). Carotenoid Pigment Accumulation in Horticultural Plants. Horticultural Plant Journal, 6(6), pp.343–360.
Islam, F., Wang, J., Farooq, M. A., Khan, M. S. S., Xu, L., Zhu, J., Zhao, M., Muños, S., Li, Q. X. and Zhou, W. (2018). Potential impact of the herbicide 2,4-dichlorophenoxyacetic acid on human and ecosystems. Environment International, Volume 111, pp. 332–351.
Jahan, F., Bhuiyan, M. N. H., Islam, Md. J., Ahmed, S., Hasan, Md. S., Bashera, M. A., Waliullah, Md., Chowdhury, A. N., Islam, Md. B., Saha, B. K., et al. (2022). Amaranthus tricolor (red amaranth), an indigenous source of nutrients, minerals, amino acids, phytochemicals, and assessment of its antibacterial activity. Journal of Agriculture and Food Research, 10, p.100419.
Peterson, M. A., McMaster, S. A., Riechers, D. E., Skelton, J. and Stahlman, P. W. (2016). 2,4-D Past, Present, and Future: A Review. Weed Technology, 30(2), pp.303–345.
Poole, I. and Kürschner, W. M. (1999). Stomatal density and index: The practice. In: Fossil Plants and Spores: modern techniques. London : The Geological Society London. pp.257–260.
Proctor, M. H. (1963). Some steps in the degradation of naphthalene acetic acid. Plant and Soil, 18(3), pp.338–345.
Rastogi, A. and Shukla, S. (2013). Amaranth: A New Millennium Crop of Nutraceutical Values. Critical Reviews in Food Science and Nutrition, 53(2), pp.109–125.
Sarker, U. and Oba, S. (2019). Protein, dietary fiber, minerals, antioxidant pigments and phytochemicals, and antioxidant activity in selected red morph Amaranthus leafy vegetable. Elfalleh, W. (Ed). PLOS ONE, 14(12), 0222517.
Shuai, H., Meng, Y., Luo, X., Chen, F., Zhou, W., Dai, Y., Qi, Y., Du, J., Yang, F., Liu, J., et al. (2017). Exogenous auxin represses soybean seed germination through decreasing the gibberellin/ abscisic acid (GA/ABA) ratio. Scientific Reports, 7(1), p.12620.
Sybilska, E. and Daszkowska-Golec, A. (2023). A complex signaling trio in seed germination: Auxin-JA-ABA. Trends in Plant Science, 28(8), pp. 873–875.
Todd, O. E., Figueiredo, M. R. A., Morran, S., Soni, N., Preston, C., Kubeš, M. F., Napier, R. and Gaines, T. A. (2020). Synthetic auxin herbicides: finding the lock and key to weed resistance. Plant Science, 300, 110631.
Uddin, M., Chishti, A. S., Singh, S., Bhat, U. H., Singh, S. and Khan, M. M. A. (2023). Effect of GA3 and NAA on growth, physiological parameters, and bioactive constituents of Ammi majus L. Industrial Crops and Products, 194, 116328.
Urry, L. A., Cain, M. L., Wasserman, S. A., Minorsky, P. V. and Reece, J. B. (2016). Campbell Biology. 11th ed. New York : Pearson Education.
Wu, M., Wu, J. and Gan, Y. (2020). The new insight of auxin functions: transition from seed dormancy to germination and floral opening in plants. Plant Growth Regulation, 91(2), pp.169–174.
Zuanazzi, N. R., Ghisi, N. D. C. and Oliveira, E. C. (2020). Analysis of global trends and gaps for studies about 2,4-D herbicide toxicity: A scientometric review. Chemosphere, 241, 125016.
Article Metrics
Refbacks
- There are currently no refbacks.
Ilmu Pertanian (Agricultural Science) ISSN 0126-4214 (print), ISSN 2527-7162 (online) is published by Faculty of Agriculture Universitas Gadjah Mada collaboration with Perhimpunan Sarjana Pertanian Indonesia (PISPI) and licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.












_2025_-_kecil_.png)
